
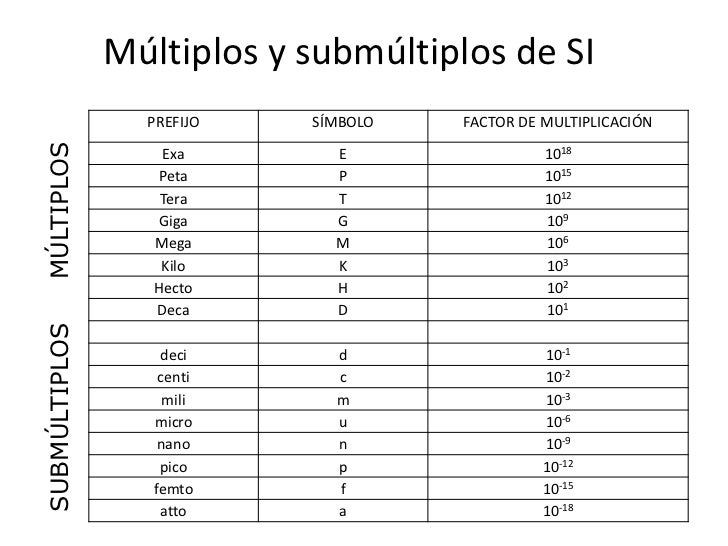
In which of the following pairings of metric system prefix and power of ten is the pairing incorrect? A) kilo- and 10–3 B) micro- and 10–6 C) deci- and 101 D) more than one correct response E) no correct response 22.

How many joules of heat are required to raise the temperature of a 95.1 g Al bar from 22.8✬ to 51.0✬? The specific heat of Al is 0.908 J/g ✬. Which of the following would be a correct set of units for specific heat? A) J/g B) J/☌ C) J/g ☌ D) J ☌/g 20.

D) A Celsius degree and a Kelvin degree are equal in size. C) A Fahrenheit degree is larger than a Kelvin degree. B) A Fahrenheit degree and a Celsius degree are equal in size. Which of the following comparisons of the size of a degree on the major temperature scales is correct? A) A Kelvin degree is larger than a Celsius degree. What is the mass, in grams, of 37.5 mL of a liquid if its density is 0.71 g/mL? A) 5.3 B) 27 C) 5 D) 266 18. If object A weighs 6.0 grams and has a volume of 3.0 mL and object B weighs 9.0 grams and has a volume of 2.25 mL, A) B is less dense than A B) A and B have equal densities C) B is twice as dense as A D) B is four times as dense as Aġ7. The density of an object is the ratio of its A) length to volume. How many conversion factors can be derived from the equality 1000 years = 1 millenium? A) two B) three C) four D) an unlimited number 15. According to dimensional analysis, which of the following is the correct set-up for the problem "How many milligrams are there in 58 kilograms?" ⎛ 1 g ⎞ ⎛ 1 mg ⎞ A) 58 kg × ⎜ 3 ⎟ × ⎜ -3 ⎟ ⎝ 10 kg ⎠ ⎝ 10 mg ⎠ The correct answer obtained from adding the measurements 7.0, 2.93 and 5.375 contains A) 2 significant figures B) 3 significant figures C) 4 significant figures D) 5 significant figuresġ2. Given the operational rules governing significant figures this answer A) is correct as written B) should be rounded to 290.6 C) should be rounded to 291 D) could be written as 2.9 × 102 11. The calculator answer obtained from multiplying the measurements 52.36 and 5.55 is 290.60. Which of the following would involve an exact number? A) the length of a car B) the mass of a sack of potatoes C) the number of feet in a meter D) the surface area of a bathroom mat 8. In which of the following cases is the given number correctly rounded to three significant figures? A) 861,000 becomes 861 B) 0.07735 becomes 0.0773 C) 75.05 becomes 75.1 D) 80.500 becomes 80,500 7. In which of the following pairs of numbers does each member of the pair contain the same number of significant figures? A) 69.0 and 69.00 B) 700.0 and 70 C) 0.07390 and 0.0739 D) 0.000095 and 950,000Ħ. In which one of the following measure numbers are all of the zeros significant? A) 0.0511 B) 2,800,000 C) 0.000840 D) 7.692600 5. To what decimal position should a measurement be recorded if the smallest markings on the measurement scale are tenths of a meter? A) to the closest meter B) to the tenths of a meter C) to the hundredths of a meter D) to the thousandths of a meter 4. In which of the following sequences are the metric system prefixes listed in order of decreasing size? A) mega giga kilo B) nano micro milli C) giga hecto centi D) deci hecto kilo 3. The “mathematical meaning” associated with the metric system prefixes centi, milli, and micro is, respectively, A) 10–2, 10–4, and 10–6.


 0 kommentar(er)
0 kommentar(er)
